Efficacy in Clinical Trials
WOXheal® - Phase II trial
It was multicentre, randomized, parallel group, active-controlled clinical trial [2008- 09] at 4 centers I.e. AIMS, Kochi, LTMG, Sion, KEM Hospital, Parel, Talwalkar Polyclinic, Matunga.
Study Drugs:
Test Drug: DPOCL
Active-Control drug : Isotonic normal saline 0.9%
Duration: 10 weeks
Objective:
Primary- percent reduction in wound area
Secondary- time to achieve complete wound closure and the total wound evaluation
Safety variables:
Regular safety evaluations, such as vital signs and hematological, biochemical parameters and adverse events were monitored & compared
Type of Ulcers:
Cutaneous (neuropathic) ulcer ranging in size between 1 and 15cm [longest single dimension], present for at least 4 weeks and classified as per Wagner's scale Grade 1 and 2 in patients with diabetic mellitus.
Application schedule - Once Every day
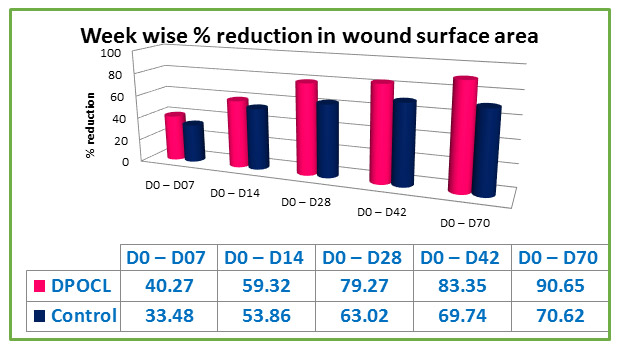
Results:
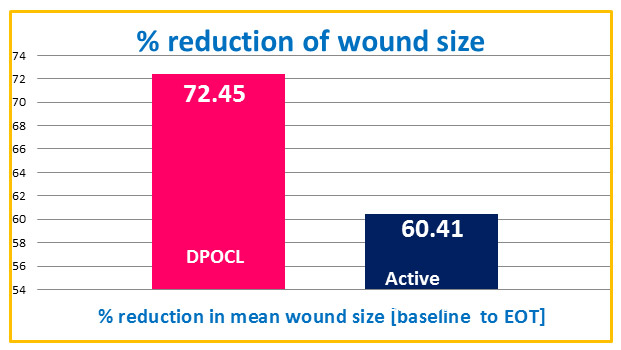
There was 72.45% wound reduction with DPOCL vs 60.40% wound reduction with active control.
Safety:
One adverse event noted in the entire duration of the trial in the "Test arm" - itching on both lower limbs (resolved without any treatment)
No adverse event at the wound site (AE was not associated with the study drugs)
Safety profile of the test was comparable with the active-control
Both the treatments were found tolerable in our patient population
Conclusion:
The Test drug DPOCL was significantly effective in the treatment of neuropathic, chronic, cutaneous ulcers of the lower extremity in patients with diabetes
The efficacy of the test drug DPOCL was significantly superior to the active control drug (NaCl) in terms of percent reduction in mean wound surface area (p<0.05)
All the patients, who received DPOCL in the phase II trial healed completely as compared to active-control
The safety of DPOCL was comparable with "Active-control - [NaCl]" as no adverse events were noted.
WOXheal® - Phase III trial
In phase III clinical trial, the efficacy and safety of DPOCL topical solution was been investigated in over 280 patients in Eastern, Sourthen, Western and Northern India, in patients suffering from diabetic foot ulcer in comparison with active-control solution i.e. isotonic normal saline (0.9 %). From the study, following results were obtained:
Efficacy Parameters
Primary efficacy parameters: Percentage of patients achieving complete wound closure
Secondary efficacy parameters Time taken for achieving complete wound closure from the onset of wound, Percentage reduction in the wound surface area at each visit
Safety Parameters
Any subject who has received at least one application of study drugs will be evaluated from point of safety.
Results
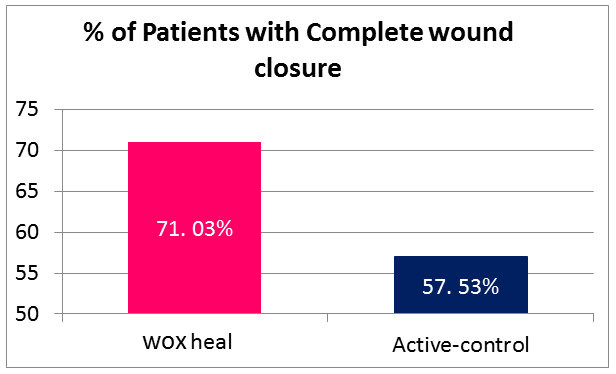
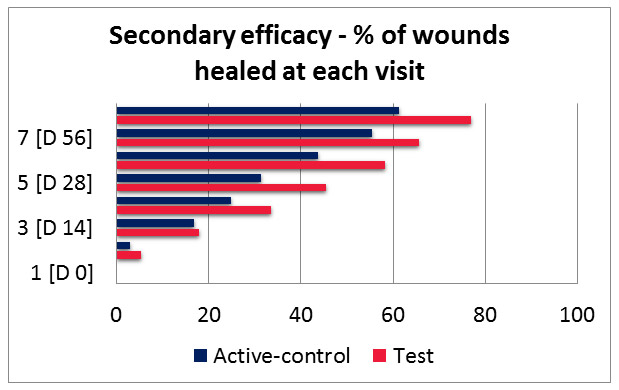
1. Complete wound healing: 71.03 % wound completely healed in DPOCL topical solution group as compared to only 57.53% in active control. This figure was statistically significant [p=0.0156]
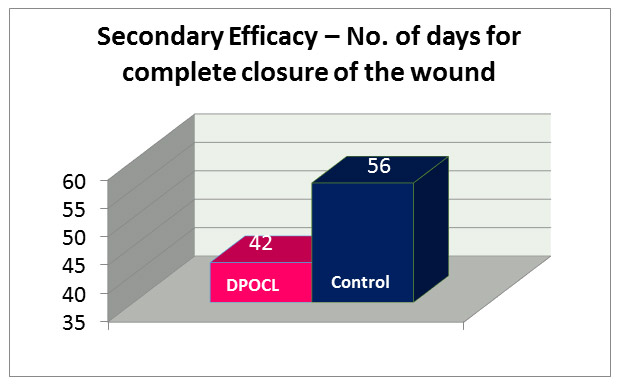
2. Time taken for complete closure of wounds: DPOCL topical solution resulted in faster wound healing compared to active-control, as median line taken for days as compared to active-control, as median time taken for complete closure of wounds in DPOCL topical solution group was 42 days as compared to 56 days in active control group
3. Overall treatment responders: More than 90% of the patient treated with DPOCL had positive response compared to 66% of active-control [treatment response defined by at least 50% wound reduction in 4 weeks.
Adverse events:
During the conduct of the trial, total six AEs were recorded following administration of investigational product, irrespective of the nature of the treatment administered [test or active control].Four out of 6 AEs did happen in the test arm and two out of 6 AEs did happen in the active-control arm. The tabulated data of AE are displayed in the following table:
WOXheal conclusion:
The preclinical and clinical trial data has proved that DPOCL is effective and safe in the treatment of diabetic foot ulcer in terms of two most important treatment goals of DFU treatment i.e. obtaining expeditious complete wound closure and infection control.
Excellent safety of woxheal is proved due to lack of AE/ SAE during clinical trials.
Due to no drug-drug or drug-lab interaction, DPOCL can be safely co-prescribed/ used with OHA or any other concurrent medications.
DPOCL is non-irritating and without any allergic or hypersensitivity or photosensitivity over entire treatment period.
Unlike other growth factors used in therapy, DPOCL did not cause overgrowth while achieving complete wound closure.
Helpline Number: +91 750 687 5056
Copyright © 2020 Centaur Pharmaceuticals
